.
--> Bacterin and toxoid vaccines
--> Conclusion
.
PORCINE PLEUROPNEUMONIA
GOOD PROTECTION WITHOUT STRESS: COGLAPIX®
By Dr Eric Brunier, Regional Market Manager – Swine, Ceva Animal Health Asia Pacific
.
BACTERIN AND TOXOID VACCINES
A new kind of vaccine associates the Apx of containing all the adhesion antigens of importance. But these vaccines still present a high level of PVR. These undesirable and stressful reactions are due to the choice of the adjuvant and the level of lipopolysaccharids (LPS) present in the vaccine.
One of these vaccines, COGLAPIX® from Ceva Santé Animale, beside the efficiency on the clinical signs and the impact of the performances, has succeeded to overcome this obstacle for a good vaccination practice. With a high quality aluminium hydroxide adjuvant combined with a well controlled level of LPS, COGLAPIX® shows a low level of post vaccination reactions.
Field trials done recently in Asia (in Thailand and in the Philippines) have confirmed this improvement under the Asian husbandry conditions. Farms with endemic APP have been selected. A trial has been done in benchmarking with a reference commercial vaccine containing the three toxoids Apx I, Apx II, Apx III and an OMP of 42 kDa (vaccine RV). Before any trial starts, the presence of APP has been confirmed by using the test ELISA Chekit Apx IV® from IDEXX.
^ Top page
.
CASE No. 1
In Thailand, the trial was done in a farrow to finish farm of 7,000 sows. A batch of 600 piglets was divided in two batches of 300 piglets each, after weaning. The animals were raised in one room (closed system with cooling).
The pigs were vaccinated at 8 and 10 weeks of age. The test batch was vaccinated with COGLAPIX®, the control batch with RV. The parameters measured were the post vaccination reactions (PVR) on all the pigs; the performances (growth and feed conversion, controlled on 2 x 120 pigs) and the lung lesions at slaughter (2 x 50 pigs). On a clearly identified subgroup of 15 pigs per batch, the rectal temperature was measured at 1 hour, 3 hours and 24 hours after each vaccination.
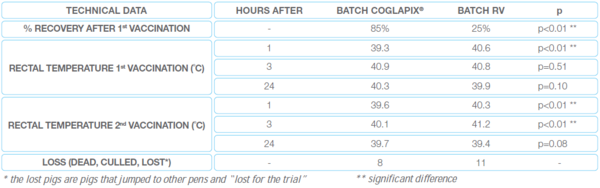
Table 1 The efficiency on the clinical signs of the trial 1 in Thailand
Results
PVR: the farm had implemented all the measures recommended to reduce the PVR. No shock has been noticed. In the batch vaccinated with RV however, 3 hours after vaccination, at the feed distribution, only 25% of the animals had recovered and were going to eat while 85% had recovered in the batch vaccinated with COGLAPIX® (the difference is significant p < 0.01). The temperature control highlight this fact even more: the pigs vaccinated with COGLAPIX® have on average a lower body temperature than the pigs vaccinated with RV (significant difference p < 0.01). (Graph 1)
Graph 1: Body Temperature After 2nd Vaccination
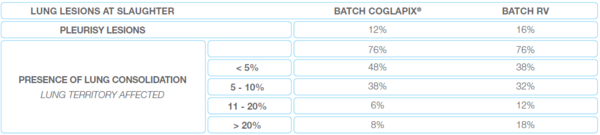
The lung lesions: are comparable between both batches except for one parameter: the severity of lung consolidation (Table 2). These lesions were less serious on animals from the COGLAPIX® batch than on animals from the RV batch.
Table 2 The lung lesions at slaughter of the trial 1 in Thailand
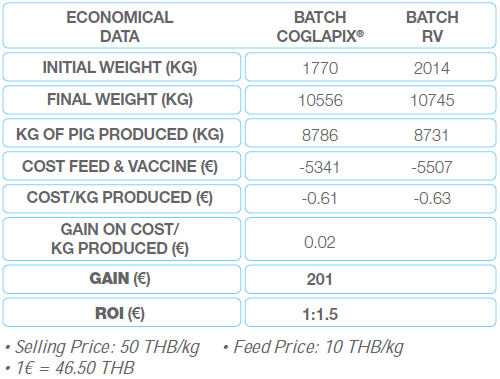
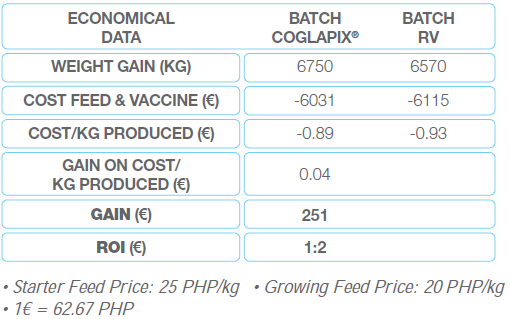
Economical results: they have been defined by comparing the cost per kg produced. Whatever the selling price, the benefit done is the cost difference between both batches (Table 3). The piglets from the COGLAPIX® batch provided a slightly better production cost to the farmer of 201 €. For 1 € invested in COGLAPIX®, the farmer earned 1.5 € more than by using the reference vaccine.
Table 3 Economical data of case No.1 in Thailand
^ Top page
.
CASE No. 2
This trial was monitored by Pr Romeo Sanchez Jr. – Associated Professor – CMV University of the Philippines – Los Baños; and his doctorate students.
The trial was done in a farrow to finish and breeding farm of 500 sows. The trial conditions and protocol were exactly the same as in the case No 1. This farm has met recent phases of clinical APP in previous batches.
In one room, 200 weaned piglets of five weeks of age were randomly shared in two batches of 100 piglets each. In each batch, one pen of 10 piglets was selected in order to measure the rectal temperature at each vaccination time during 48 hours. The piglets were vaccinated in the tested batch with COGLAPIX® and in the controlled batch with the same reference vaccine, as in the case No. 1, that we call: RV. The vaccination was done at 6 and 9 weeks of age.
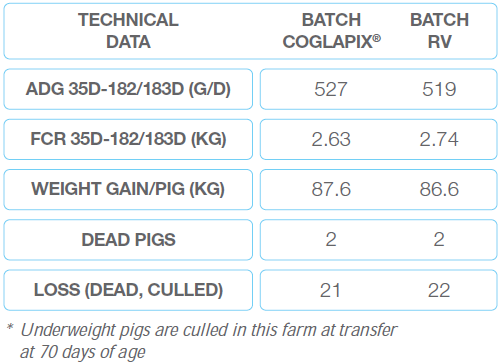
They stayed in the building until 70 days before being transferred to the growing-finishing facility. The batches, identified by different ear tags, were not mixed during the transfer. The farm was used to cull the underweight piglets at the transfer. 20 piglets of the COGLAPIX® batch and 21 piglets of the RV batch were culled.
Table 4 Case No.2 In Philippines
Results
PVR: The farm did not take any special measures to prevent the PVR. No pig died from the vaccination but some post vaccination shocks (tremor, vomiting, convulsion, generalised erythema – Photo 1) were noticed in the RV batch only: 2 pigs after the first vaccination; 1 pig after the second vaccination. The batch COGLAPIX® did not show any PVR at all.
Photo 1: Pig in PVR; erythema
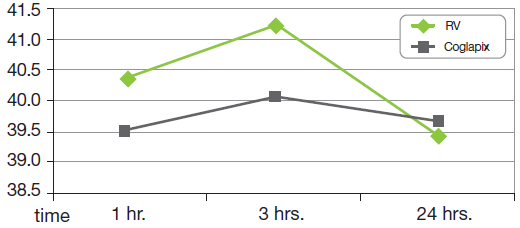
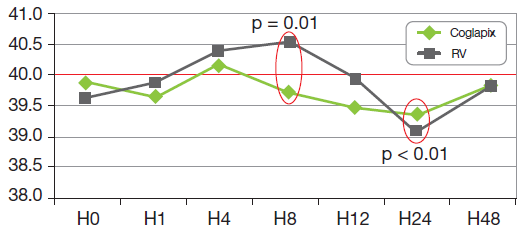
The rectal temperature control showed a net difference with a lower level and a shorter time over 40oC for the batch COGLAPIX® (graph 2).
Graph 2: Rectal Temperature After 1st Vaccination (H0)
Lung lesions: At slaughter, the batch COGLAPIX® had a better lung lesion score (Table 5). In this group, there was no lesion of pleurisy when 4% of the lungs showed pleurisy in the RV group.
Table 5 Lung scoring of the trial 2 in Philippines
Economically, the COGLAPIX® batch had a slightly better cost of 375 € less then the RV batch which means a return of investment of 1:2 (1 € invested in COGLAPIX® brings 2 € more to the farmer compared with the RV vaccine). (Table 6).
Table 6 The impact of the performance of the trial 2 in Philippines
*ADG: average daily gain
**FCR: feed conversion rate
^ Top page
.
CONCLUSION
The protection of a farm against APP may combine different solutions including husbandry management measures concerning the control of the introduced animals, reasoned antibiotic use for short treatment periods only, and vaccination.
Different vaccines exist. The bacterin vaccines are very specific to a small number of serotypes. The toxoid vaccines containing the 3 Apx antigens allow a protection against all serotypes. Unfortunately, many vaccines are responsible for post vaccination reactions that worry the farmer and make him more reluctant to apply a vaccination policy.
COGLAPIX® provides the advantages of a universal vaccine against all serotypes but without the PVR. Trials done in the Asian field in APP contaminated farms in benchmarking with a reference vaccine containing the three Apx and an OMP 42 kDa, have confirmed that COGLAPIX® brings a true difference with the absence of PVR. At the same time the economical data were slightly better than the reference vaccine and, in each case studied, showed a better return on investment between 1 : 1.5 to 1 : 2 with COGLAPIX®.
^ Top page
.
(Source: "Axis Special Swine Issue - Asia Pacific” - November 2009)
.
<< Back to Porcine Pleuropneumonia
<< Back to Disease Informations
Related topics: trial coglapix disease app vaccination protection swine result

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam