.
--> Introduction
--> Results
.
APP: COMPARISON OF FOUR DIFFERENT VACCINES UNDER FIELD CONDITIONS IN THE PHILIPPINES
By Dr Eric Brunier, Regional Market Manager – Swine, Ceva Animal Health Asia Pacific
E. Brunier*, T. Plete**, R. Bijasa***, C. Ignacio, M. Irorita, R. Sanchez**, C. Hernandez
* Ceva Animal Health Asia Pacific, 3.06, Level 3, Wisma Academy, Lot 4A, Jalan 19/1, 46300 Petaling Jaya Selangor, Malaysia
** College of Veterinary Medicine, University of the Philippines, Los Banos, Laguna 4031, Philippines
*** Ceva Animal Health Philippines, E-1605 East Tower, Philippine Stock Exchange Center, Exchange Road, Ortigas Center, Pasig City, Philippines
.
INTRODUCTION
Coglapix® - the vaccine against Porcine Pleuro-Pneumoniae (PPP) due to Actinobacillus pleuropneumoniae (App) has been developed by Ceva Santé Animale to protect the vaccinated animals against the clinical signs and to maintain the performance in a contaminated environment with App but without presenting the undesirable post vaccination reactions often met with the toxoid vaccines present on the market. This vaccine is a toxoid vaccine, containing bacterins from whole bacteria bodies too and especially balanced in lipo-polysaccharids to limit the reactogene properties of these molecules. A trial under field conditions has been conducted in the Philippines in comparison versus two toxoid vaccines and one bacterin vaccine of the market.
.
MATERIAL AND METHOD
The trial was done in a 550-sow farrow-to-finish farm located in the Philippines. This farm has been experiencing acute outbreaks of App for five months. No vaccination was done prior to the trial. Before starting the trial, the presence of App has been checked with the kit IDEXX CHEKIT® Apx IV on 15 sows from parity 1 to 5 and on 15 pigs between 5 to 6 months of age. 80% (12/15) of the sows and 33% (5/15) of the hogs were positive.
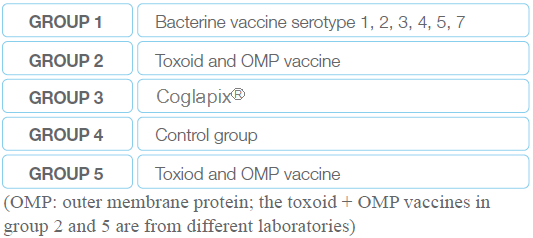
300 piglets of 5 weeks of age have been allocated in 5 groups (4 pens of 60 pigs per group). They have been vaccinated at 6 and 9 weeks of age with a different vaccine per group (Table 1); the pigs of the control group have been injected with a saline solution.
Table 1 A different vaccine in each group
The performance has been noted until slaughter: individual weight, total feed consumed (only from 60 days old). 20 pigs from each group have been observed at slaughter and a lung scoring has been realised (scoring on 28 per lung). Among each group, one pen has been selected and the pigs have been monitored for PVR after each vaccination, their rectal temperature has been measured.
^ Top page
.
RESULTS
Clinical scores
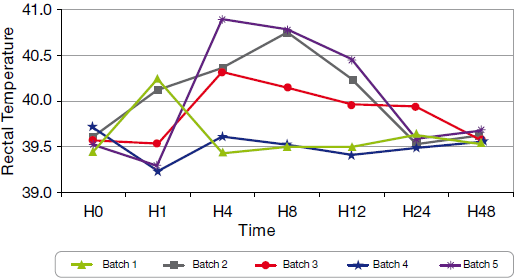
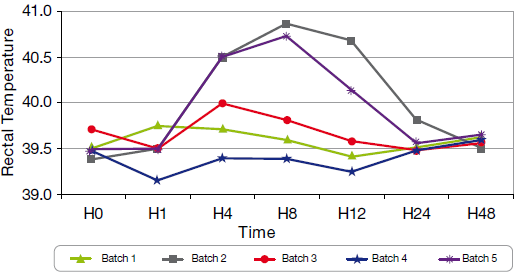
Post-Vaccination Reactions (PVR) have been observed in groups 2 and 5. In group 2, two pigs after each vaccination have shown dyspnea, depression, vomiting and skin discoloration. In group 5, one pig has shown incoordination and hypersalivation. Besides the marked PVR, clinical score as nasal discharge, coughing, dyspnea, depression, anorexia have been noted (1 point/ sign). The temperature measurement has shown significant differences between the toxoid + OMP vaccines on one hand, and the new toxoid vaccine and bacterin vaccine on the other hand. The time spent over 40°C is longer for groups 2 and 5.
Graph 1 Rectal temperature 1st shot
Graph 2 Rectal temperature 2nd shot
^ Top page
.
Performance
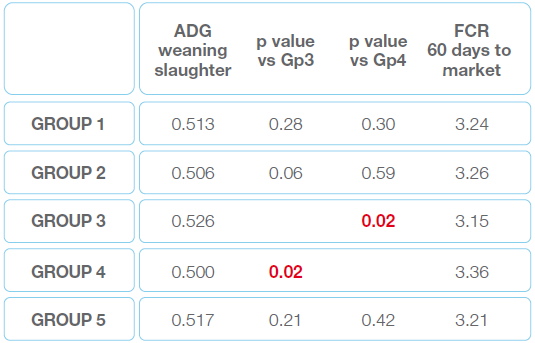
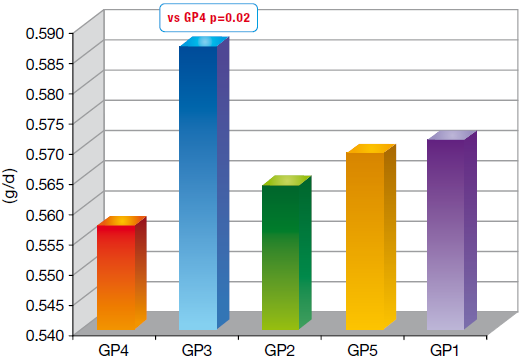
The average daily growth (ADG) as well as the feed conversion rate (FCR) have been measured as shown in Table 2.
Table 2 ADG và FCR in 5 groups
Graph 3 ADG Weaning Slaughter
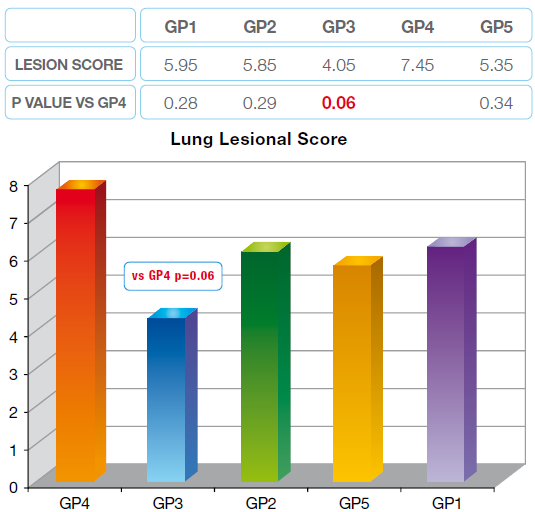
Group 3 had a better performance. That’s the only group to have a significant difference with the control group (4). The same trend has been observed for the lesion score.
Graph 4 Lung Lesion Score in 5 groups
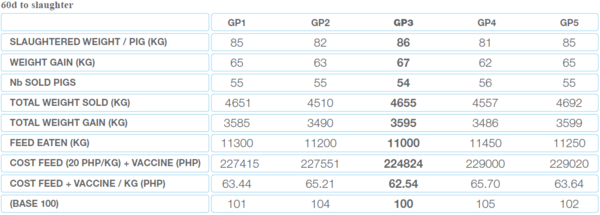
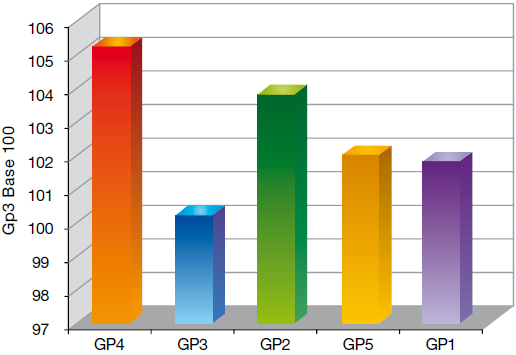
Economically, group 3 allowed the farmer to get the best return on investment as is shown by calculating the cost to grow (cost/kg of pig produced). The lower the cost, the higher the benefit.
Table 3 The cost to grow in each group
Graph 5 Cost/kg of Pig produced
^ Top page
.
DISCUSSION AND CONCLUSION
At the opposite of the previously existing toxoid vaccines (group 2 and 5), Coglapix® (group 3) has shown no PVR. This fact is corroborated by a shorter time under 40ºC. Reactogene vaccines induce indeed a higher body temperature after vaccination (Hypersensitivity). The bacterin vaccine (group 1) hasn’t shown PVR either. The body temperature shows a difference with the control group (Group 4) only one hour after vaccination. The performance and lung lesion score have been significantly improved versus the control batch only for the tested vaccine Coglapix®; this despite the fact that the App pressure was moderate (Positivity to IDEXX CHEKIT® Apx IV) without clinical outbreak (including the control group).
In this trial, Coglapix®, the toxoid vaccine tested from Ceva Santé Animale, has met its claims: less PVR and improved performance.
^ Top page
.
Any bibliographic reference can be communicated by contacting Dr Eric BRUNIER at eric.brunier@ceva.com
(Source: "Axis Special Swine Issue - Asia Pacific” - November 2009)
.
<< Back to Porcine Pleuropneumonia
<< Back to Disease Informations
Related topics: toxoid effect toxoid vaccine app actinobacillus pleuropneumoniae vaccine compare experiment swine

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam