...
--> Brief history on the SG 9R-strain
--> Infection and immune-protection mechanism of Salmonella
--> The Salmonella Gallinarum 9R-strain as a vaccine for chickens
...
SALMONELLA GALLINARUM 9R STRAIN AS VACCINE
By Edir N. Silva – Campinas, SP. – Brazil
.
BRIEF HISTORY ON THE SG 9R-STRAIN
The rough (R) strain of Salmonella Gallinarum (SG), named as 9R, was developed in England from a number 9 field virulent smooth strain, with a paper published by Smith in 1956, based on the knowledge that rough mutants lose their virulence capability. Many publications have proved its safety as a vaccine, non reverse in virulence, and ability to protect chickens against fowl typhoid (FT). Since then, it has been used extensively in many countries where the FT is endemic, mainly in brown layers, but also in white egg layers and breeders.
One of the greatest advantages of the use of the SG 9R vaccine is that it gives good protection and does not interfere with the tests used for pullorum-typhoid control. Besides controlling FT, the SG 9R vaccine has been used in several countries as an additional tool in the control of Salmonella Enteritidis (SE) in commercial layer flocks. Better immunity, lower cost in comparison with kill products, no local reactivity, are its strongest points.
.
INFECTION AND IMMUNE-PROTECTION MECHANISM OF SALMONELLA
Fowl typhoid (FT) in chickens and turkeys is caused by Salmonella enterica, subspecie enterica, serovar Gallinarum (S. Gallinarum) and, in its acute form, is almost exclusively a septicaemic disease, more observed in the later growing period, and in mature stock; although age is not a limiting factor for disease occurrence (Berchieri, et al. 2001). The severity of FT is highly genetically dependent. The brown egg layer breeds are very susceptible, while the white leghorn types are quite resistant, but still carry the organism for a long period of time (Berchieri Jr., et al. 2000).
A rough mutant (R) strain of S. Gallinarum (SG), named as 9R, was developed in England from a field virulent smooth strain number 9 (Smith, 1956), and used as a vaccine worldwide.
It is known that the infection with 9R results in a mild systemic infection, not prolonged, leading to both cellular and humoral immune responses, which peak soon after bacterial clearance. The bacterial clearance after vaccination occurs at three weeks post infection, and coincides with increases in circulating anti-Salmonella antibodies, increases in T cell proliferation and the expression of interferon gamma. There is no persistence of the vaccine strain in the gastrointestinal tract (Silva, et al., 1981; Wigley, et al., 2005).
Although, the immune response to SG, either infection or live vaccination, is poorly characterized (Jones, et al., 2001), it has been demonstrated that protection could be related to outer membrane proteins (OMP).
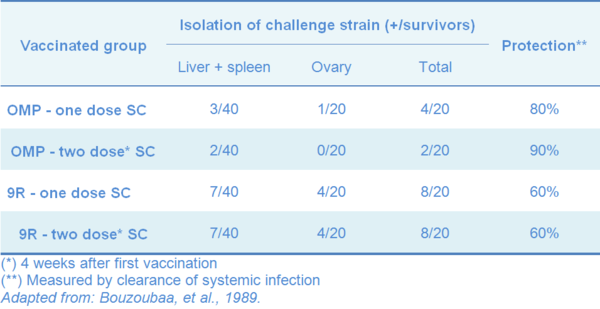
Subcutaneous inoculation with purified OMP from SG field strain, gave better protection results in chickens experimentally challenged with the wild type of SG, in comparison with 9R subcutaneously (SC) vaccinated ones (Table 1). The protection was measured by clearance of challenge organism from internal organs (Bouzoubaa, et al., 1989).
Table 1 Outer membrane protein (OMP) of Salmonella Gallinarum and 9R strain as vaccines for fowl typhoid in chickens
^ Top page
.
THE SALMONELLA GALLINARUM 9R-STRAIN AS A VACCINE FOR CHICKENS
The SG 9R Vaccine
The SG 9R vaccine is a suspension of the live avirulent stable rough strain of SG. It is the most commonly used vaccine for FT (Harbourne et al., 1963) presented in the freeze-dried form.
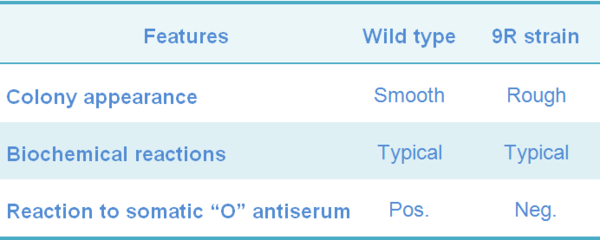
The 9R-strain does not contain the somatic antigen characteristics of the smooth forms of SG, but it shows the same biochemical reactions characteristics of smooth virulent SG strains. However, 9R-strain suspensions, from broth or agar culture, are rough when examined by the acriflavine slide test.
Table 2 Characteristics of the Salmonella Gallinarum strains
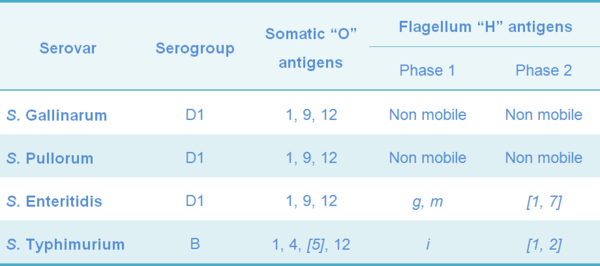
The SG is a non mobile serovar (they do not have flagellum), belonging to the Salmonella serogroup D1, with somatic antigens 1, 9 and 12, the same as in other pathogenic strains for chickens such as S. Enteritidis (SE) and S. Pullorum (SP). So, cross protection is expected between them (Table 3). The SG 9R vaccine has being used in several countries as an additional tool in the control of SE in commercial layer flocks (Feberwee et al., 2001).
Table 3 Classification of the most important poultry pathogenic Salmonella
^ Top page
.
Use of the SG 9R-Strain Vaccine in Chickens
The use of the SG 9R vaccine for FT must be done before field challenge occurs, and it should be considered in a FT endemic area. Due to it cross reactivity it has been used as a tool to control SE infection in layers and breeders.
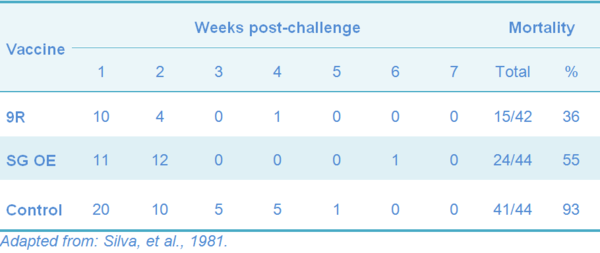
Vaccination reduces flock losses due to FT, contributes to the reduction of SE infection and egg transmission in infected flocks, but still the field infection can still occur (Table 4 and 5).
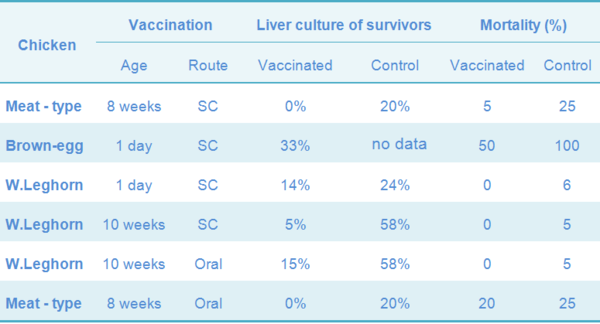
Table 4 Morbidity and mortality of chickens vaccinated with 9R and challenged with a pathogenic strain of S. Gallinarum four weeks later
Table 5 Mortality following challenge of one-year-old meat-type chickens vaccinated subcutaneously with 9R and oil-emulsion (OE) vaccine
There are some indications that the use of the 9R vaccine can reduce the shedding of non related serogroup as S. Typhimurium (Table 6).
Table 6 Protection of 10 weeks-old W.Leghorn subcutaneously vaccinated (107 CFU/dose) with 9R vaccine and orally challenged six weeks later with Salmonella Typhimurium (109 CFU/bird)
Vaccination should be considered as part of an FT and SE eradication program.
^ Top page
.
Vaccination: Doses, Age and Route of Administration
The vaccine titer is important. The dose used per bird is between 106 to 107 colony forming units – CFU (OIE, 2004).
Injections, either SC or intramuscular, are the preferable vaccination routes. Two doses protects better than one single vaccination. Water vaccination has been used in many situations, but it is less protective. See Table 4 (Bouzoubaa, et al., 1989; Silva, et al., 1981).
It is usual to vaccinate at 6-8 weeks and again at 14-16 weeks of age. Field data has demonstrated that broiler chicks vaccinating at day-old at the hatchery plant level, does not interfere with production parameters.
Antimicrobials should be avoided before and after vaccination. A booster dose should be required, and vaccination should be avoided during laying period.
Based on the effect on body weigh gain, 4 weeks of age was recommended as the minimum vaccination age of the 9R vaccine considering the safety and efficacy of the vaccine (Lee, et al,. 2005).
^ Top page
.
Transmission of the SG 9R-Strain
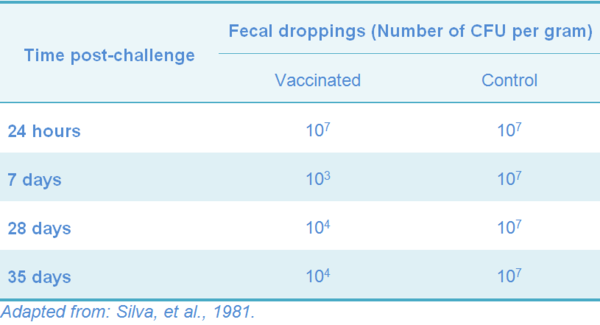
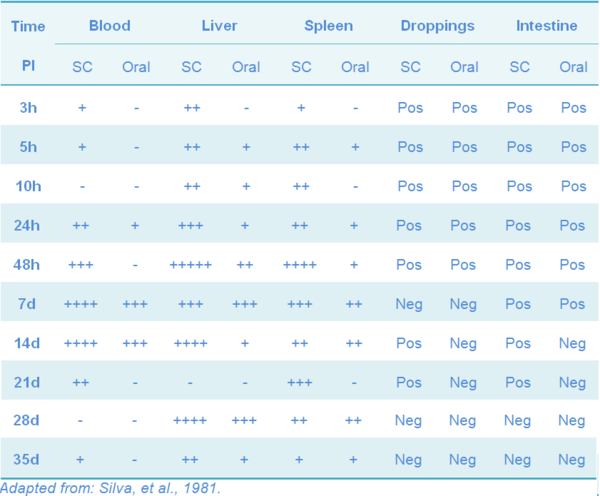
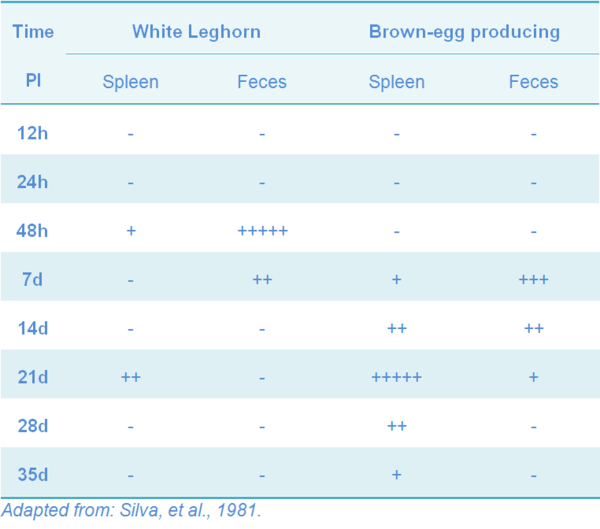
Vaccinated chickens can harbor the strain for a short period of time, and the length depends on the age and breed (Tables 7 and 8), as is seen both in experimental SG infection and vaccination (Berchieri, et al., 2005; Silva, et al., 1981).
Table 7 Persistence of 9R strain in meat-type chickens vaccinated at day of age by subcutaneous (SC) and oral routes
Table 8 Persistence of 9R strain in two different breeds of chickens vaccinated at day of age by subcutaneous route
The 9R-strain can be egg transmitted in a few cases if applied by injection during egg production period, but it is rare. The 9R-strain was detected only in small numbers in the first weeks post-vaccination, at a prevalence level of 1% with 95% CI (Feberwee, et al., 2001). In another study, the vaccine strain could not be isolated from any of the 450 pools of 10 eggs, confirming the indication of its rare spread to the egg content (Feberwee, et al. 2001a). Even in experimentally FT infected flocks, egg contamination is rarely found (Berchieri, et al., 2005).
The horizontal transmission of the SG-9R strain has not been proved. In a field study, the potential spread of the vaccine strain from vaccinated flocks to non-vaccinated flocks has been studied after both the primary and the booster injection at four different rearing farms and at one layer farm. The vaccinated and the non-vaccinated flocks were monitored at regular intervals by bacteriologic and serologic examination. No evidence was found for the fecal spread of the vaccine strain (Feberwee, et al. 2001). Experimentally, meat type chickens can excrete the 9R-strain by the feces for up to five weeks after day-old vaccination; excretion was reduced to 24h post-vaccination when chicks were vaccinated at 8 wks of age, but was not detected in 10 weeks old white Leghorns, nor in one-year old meat type vaccinated by SC (Silva, et al., 1981).
^ Top page
.
Vaccination with SG 9R in Layer-Chickens
Some simple measures can be taken in FT infected flocks to help control the disease. For example, the removal of dead birds quickly from the chicken house seems to prevent and reduce the FT in the flock (Oliveira, et al. 2005).
Besides controlling FT, the SG 9R vaccine has been used as an additional tool in the control and in the reduction of SE in commercial layer flocks, but is not expected to confer complete protection against infection in the field (Feberwee et al., 2001).
In a field study in the Netherlands, 80 commercial layer flocks from an area of increased risk of SE infection were vaccinated with a SG 9R. The efficacy study was done by assessment of the flock level occurrence of SE infections in the vaccinated groups compared with that of a non-vaccinated groups of 1,854 flocks hatched in the same period. The occurrence of SE infections in the vaccinated group was 2/80 (2.5%), and significantly lower (P = 0.01) than that of the nonvaccinated group (214/1854 = 11.5%). Table 9 (Feberwee, et al. 2001a).
Table 9 Field trial with the occurrence of SE infections in commercial layer flocks
^ Top page
.
Serological Response to SG 9R Vaccination
Although the 9R is a rough non-mobile strain, with a lack of somatic “O”antigens, it still may stimulate the production of transient antibodies (Ab) detectable depending on the test used. There is no direct relationship between Ab titers and protection, although specific Ab may be involved (Bouzoubaa, et al, 1989; Silva, et al., 1981). The serological tests could be detecting Ag against OMP and no to somatic antigens, since all chickens vaccinated with the 9R-strain or with OMP developed antibodies detectable by the microagglutination (MAG) test, and in some vaccinated groups as many as 100% of the birds developed antibody levels detected by seroagglutination (Bouzoubaa, et al. 1989).
The MAG test is much more sensitive for Ab detection than the pullorum test. MAG test can detect Ab as early as two weeks post-vaccination (PV), with 100% of positive birds by the 7th weeks after a single subcutaneous (SC) dose. Some reactors to the pullorum test can be detected between 2 and 7 weeks post-vaccination. These serological results suggest that a period of at least seven weeks should be allowed between vaccination with 9R and testing for pullorum-typhoid (Silva, et al., 1981).
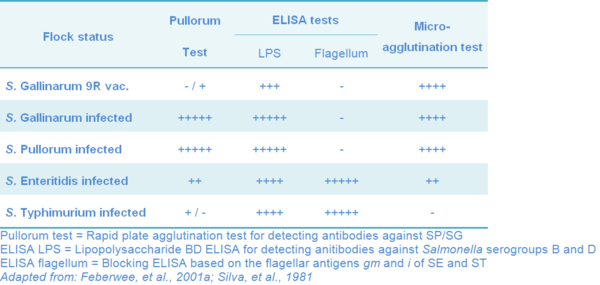
However, SG 9R vaccinated flocks can be serologically differentiated from field SG, SP, SE or S. Typhimurium (ST) infected flocks as shown in Table 10.
There are commercially available Elisa Kits to detect antibodies against Salmonella infection in chickens. The indirect LPS-BD-Elisa (ID-Lelystad, Lelystad, The Netherlands) detects antibodies against Salmonella group B and D antigens (O-antigens 1, 4, 5, 9 and 12). More specific Kits based on gm and i-DAS-Elisa are highly specific and sensitive tests for detecting antibodies against the gm and i flagellum antigens of SE and ST.
Vaccinated layers with SG 9R showed 0% positive results in the pullorum test (rapid plate agglutination test for detecting antibodies against SP/SG). When the LPS-BD-Elisa was used, the positive results were 59.0%. And, the mean specificities of two blocking ELISAs (gm- and i-double antibody sandwich ELISAs) on the same sera were 99.6% and 96.1%, respectively (Feberwee, et al., 2001a).
Table 10 Serological response of chicken to SG 9R vaccination and infected with the most prevalent avian pathogenic Salmonella strain
^ Top page
.
How to Test the Vaccine Protection in Chickens?
There is no satisfactory method of assessing the protection afforded by the vaccine in the field. However, experience has shown that the vaccine can provide some benefit in situations where control cannot be achieved by hygiene and management alone.
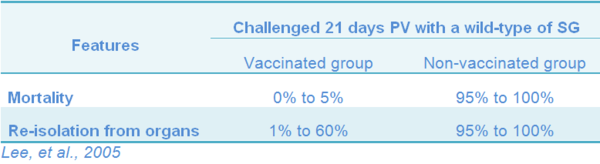
Laboratory evaluation is based on mortality and re-isolation of challenge strain (clearance rate). Vaccination of young layer hens at age of 2, 4 and 6 week-old showed protection rate against challenge with the wild-type SG observed 21 days after one-dose vaccination, as measured by mortality, was 0% to 5% in the vaccinated group, while the control not vaccinated group was 95% to 100%. In addition, the control group demonstrated a 95% to 100% re-isolation rate of the challenge strain in internal organs and the caecum, while in the vaccinated group only a 1% to 60% re-isolation rate was observed. Table 11 (Lee, et al. 2005).
Table 11 Laboratory test to evaluate 9R vaccine protection in layers hens vaccinated with one dose at age of 2, 4 and 6 weeks of age
^ Top page
.
Safety and Post-Vaccination Reaction of SG 9R-Strain
The 9R strain is not known to be pathogenic to humans, and there are no special risks associated with the manufacture or use of the vaccine.
The SG 9R-strain has been proved to be safe for chicken without reversion to virulence and spread to nontarget flocks, although mutant resistant to antibiotic has been shown to require a longer period of time to establish systemic infection (Silva et al., 1981).
Mild lesions can be seen in the liver and spleen of brown-egg and meat type chicks vaccinated at day of age, but not in white leghorn, similarly vaccinated (Silva, et al., 1981).
As the vaccine causes a transient systemic infection with replication in the liver and spleen during the first weeks post-vaccination, some degree of growth depression could be expected, but not mortality, although this is not a field observation (Silva, et al., 1981).
The vaccination at 2 weeks old of laying hens with one and 10 doses causes a small depression of body weight gain at 5 weeks, but not with chickens vaccinated with one and 10 doses at the age of 4 and 6 weeks – Table 12 (Lee, et al,. 2005), or in white leghorn (Silva, et al., 1981).
Table 12 Effect of one or 10 doses of the 9R vaccine on the body weight gain of laying hens
^ Top page
.
BIBLIOGRAPHY
Berchieri Jr., A., Oliveira, GH., Pinheiro, LAS., & Barrow, P. 2000. Experimental Salmonella gallinarum infection in light laying hen lines. Braz. J. Microbiol. 31:50-52.
Bouzoubaa, K., Nagaraja, KV., Kabbaj, FZ., Newman, JA., Pomeroy, BS. 1989. Feasibility of using proteins from Salmonella gallinarum vs. 9R live vaccine for the prevention of fowl typhoid in chickens. Avian Dis. 33:385-391.
Feberwee, A., de Vries, TS., Hartman, EG., de Wi,t JJ., Elbers, AR., de Jong, WA. 2001a. Vaccination against Salmonella enteritidis in Dutch commercial layer flocks with a vaccine based on a live Salmonella gallinarum 9R strain: evaluation of efficacy, safety, and performance of serologic Salmonella tests. Avian Dis., 45:83-91.
Feberwee, A., Hartman, EG., de Wit, JJ., de Vries, TS. 2001. The spread of Salmonella gallinarum 9R vaccine strain under field conditions. Avian Dis., 45:1024-1029.
Harbourne, JF., Williams, BM., Parker, WH. & Fincham, IH., 1963. The prevention of fowl typhoid in the field using a freeze-dried 9R vaccine. Vet. Rec., 75:858-861.
Jones, MA., Wigley, P., Page, KL., Hulme, SD., Barrow, PA. 2001. Salmonella enterica Serovar Gallinarum requires th Salmonella pathogenicity island 2 type III secretion system but not the Salmonella pathogenicity island 1 type III secretion system for virulence in chickens. Infect. and Immun., 69:5471-5476.
Lee, YJ., Mo, IP., & Kang, MS. 2005. Safety and efficacy of Salmonella gallinarum 9R vaccine in young laying chickens. Avian Pathol 34:362-366.
OIE. 2004. Fowl typhoid and pullorum disease. OIE terrestrial Manual. Chap. 2.7.5. p.868-877.
Oliveira, GH., Berchieri Jr., A., Fernandes, AC. 2005. Experimental infection of laying hens with Salmonella enterica serovar Gallinarum.Braz. J. Microbiol. 36:51-56.
Ryll, M., & Hinz, KH. 1995. [Differentiation of Salmonella gallinarum rough and smooth strains using gas chromatography analysis of their cell-bound fatty acids] [Article in German]. Berl Munch Tierarztl Wochenschr. 108:347-349.
Silva, EN., Snoeyenbos, GH., Weinack, OM., Smyser CF. 1981. Studies on the use of 9R strain of Salmonella gallinarum as a vaccine in chickens. Avian Dis. 25:38-52.
Smith, HW. 1956. The use of live vaccine in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J. Hyg. 54:419-432.
Wigley, P., Hulme, S., Powers, C., Beal, R., Smith, A., Barrow, P. 2005. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterize immunity to fowl typhoid in the chicken. Vet. Res., 2:1-9.
If you need to download this article, please do not hesitate to contact us!

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam