...
-->Introduction
+Major Contaminants of Hatching Eggs
+The Bacterial Penetration in the Eggs
-->How To Disinfect Hatching Eggs?
+Implications of Improper Sanitizing
-->Conclusion
-->Annexe I: Method Using Formalin As Fumigation (OIE – Code for Terrestrian Animals)
-->Annexe II: Major Group of Disinfectants and Characteristics
...
DISINFECTION OF HATCHING EGGS
- IMPORTANCE AND PRACTICAL ASPECTS -
By Dr Vincent TURBLIN – Deputy Regional Market Manager – Poultry
Ceva Animal Health Asia – Selangor, Malaysia
.
INTRODUCTION
Nowadays, good cleaning and disinfection practices are widely acknowledged as part of the optimization of the viable DOC production in a hatchery. Good egg hygiene has often proven to improve the viability and the quality of the DOC (Archienbuwa et Coll., 1980).
Indeed, a strong bacterial penetration through the egg shell will affect several aspects in hatching quality, like early embryonic mortality, egg yolk infection, DOC mortality before hatching, besides a significant higher mortality and heterogeneity of the chicks during the first week of age.
.
1. WHY DISINFECT EGGS?
1.1 Major Contaminants of Hatching Eggs
The most often pathogens met in hatchery are well known and belong to the following genus (Thermote L., 2003):
- Pseudomonas,
- Escherichia coli,
- Salmonella,
- Mycoplasma,
- Several moulds like Aspergillus fumigatus
^ Top page
.
1.2 Ways of Contamination
Clearly, the aforementioned micro-organisms can contaminate the hatching eggs in different ways (vertical and/or horizontal contamination).
The vertical contamination of the hatching eggs will involve the reproductive tract of the hen, including the last part of it which is shared with the digestive tract (the cloaca). Thus, this vertical transmission of the pathogens could be pedagogically divided into two different ways of contamination of the hatching eggs:
- Transovarian transmission, in which the micro-organism is already located in the ovules even before the ovulation. SP and SG are probably the most well known examples of this kind of transmission.
- Contamination of the surface of the eggshell and thus penetration of the pathogen into the hatching egg. It occurs either during the oviposition through the contact with the cloaca of infected hens or immediately after it through contact with contaminated feces and litter. SE and ST can be mentioned as examples of such ways of vertical transmission.
The horizontal contamination needs a source inside of the hatchery; a kind of reservoir from which air, water or manipulation of the eggs will spread the micro-organisms to the hatching eggs by direct contact. This is the case for E.coli, Pseudomonas and Staphyloccocus.
It is important to consider that the environment of laying house will deeply influence the shell cleanness (Widing G.P, 2000). Even the quality of the digestion of the hens can also interfere with the quality of the egg (contamination aspect besides all the other nutrition transfers to the DOC). Indeed, after oviposition, those eggs non-contaminated vertically will still remain susceptible to the health status of the breeder flock (Salmonella…) and to the pressure of bacteria responsible for hatching-egg putrefaction like Pseudomonas, which will be enhanced in diarrheic flocks.
^ Top page
.
1.3 The Bacterial Penetration in the Eggs
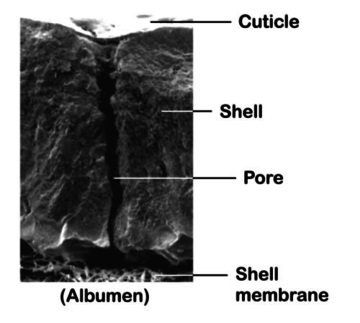
An intact cuticle is commonly acknowledged as an efficient barrier against penetration. Nevertheless, the immature cuticle of freshly laid eggs could be easily penetrated. Moreover it has to be considered that these freshly laid eggs will be in contact with tough materials on the nests or even the hens themselves can cause scratches on them, so it is a critical stage in bacterial penetration (Sparks and Burgess, 1993).
Besides, there are thousands of pores on the egg shell which are essential for exchange of gases. Most of them are covered by a cuticle preventing liquid and bacterial penetration, but several others are not and thus allowing the penetration of micro-organisms into the eggs.
Furthermore, it has also to be considered that, after the oviposition, eggs will switch from a 40°C temperature to an ambient one. During this process, they will dry and cool down, creating a pressure gap through the egg shell. In order to balance inside and outside pressure, the air will be “sucked” through the pores and therefore increasing the bacterial penetration (Bruce, J et coll., 1994, Lock, J.L., et coll., 1992).
As a result, the production of healthy hatching eggs is the result of strict sanitary prophylaxis managing all the steps, from the breeder stage to the hatchery itself.
Source: North Carolina Cooperative Extension Service Website
(http://www.ces.ncsu.edu/depts/poulsci/tech_manuals/contamination_hatching_eggs.html)
Table 1 & 2: OIE / WHO Code for Terrestrian Animals
^ Top page
.
2. HOW TO DISINFECT HATCHING EGGS?
Immediate application of the sanitizer as soon as the eggs are collected is of utmost importance. Failure to apply the sanitizer in a timely manner will give the opportunity to the bacteria to penetrate into the hatching eggs through the pores of the eggshell and thus reaching the shell membrane. Inside the eggs, the microorganisms will not be exposed to the sanitizer anymore and, during the incubation process, they will find the ideal condition to multiply in the egg's interior. Therefore, the disinfection aims at destroying the microorganisms before they enter into the egg.
2.1 Choosing the Disinfectant
The disinfectant used, in itself, must fulfill different requirements: to have a broad spectrum (to be able to destroy a wide the range of micro-organisms, from Gram -ve to Gram +ve and moulds), to be active at low concentration, safe for human users as for eggs, chemically stable, without any corrosive action on metals, and comply with local regulations.
Moreover, it is necessary to take into consideration that different factors will influence the activity of a disinfectant as the kind of surface and its status, the bacteriological and physico-chemical properties of the dilution water, the concentration and the temperature.
The major families of disinfectants are the following:
- Halogens (chlorine, iodine),
- Aldehydes,
- Quaternary Ammoniums Compounds,
- Phenol and derived,
- Peroxides
Each family has its own characteristics and their activity spectrum is relatively specific (cf Annex 2).
^ Top page
.
2.2 Way of Application
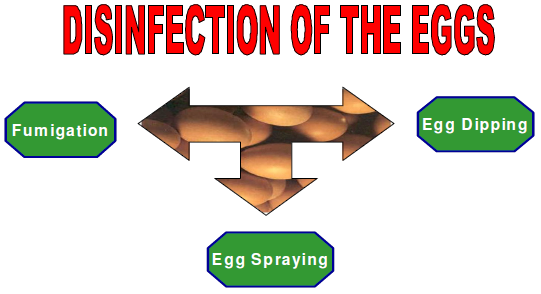
According to the sanitary code for earth animals of Office International des Epizooties / World Organization for animal health, the egg disinfection means:
- Submit the eggs to a formaldehyde fumigation,
- Apply a desinfectant for egg shell by pulverization or immersion, according to the manufacturer’s instructions,
- Or apply other methods of disinfection in accordance with veterinary authorities.
Figure 1: Three major ways of egg-disinfection in the farms
^ Top page
.
2.2.1 Fumigation
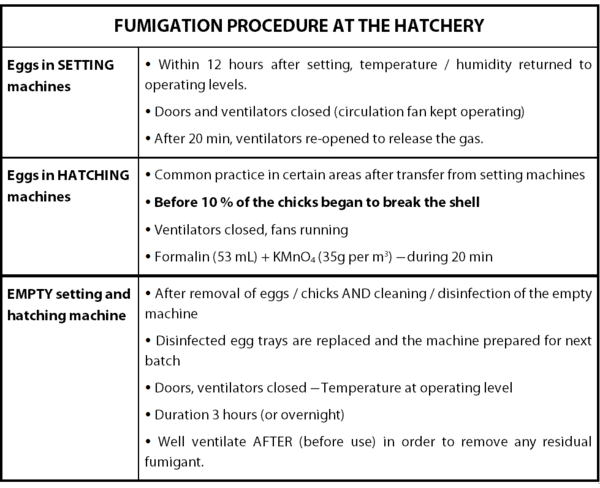
Clearly, only by exposing the hatching eggs to formaldehyde is not enough to sanitize them. In a proper fumigation procedure, five different factors have to be taken into consideration: concentration of formaldehyde, temperature, humidity, time of exposure and circulation of the gas.
Regarding to the concentration of formaldehyde, no consensus is really established currently on the optimum concentration of formaldehyde required for the sanitization of hatching eggs. Usually, three levels of concentration have been used and two different methods have been adopted (cf. Annex 1).
As the formaldehyde gas rapidly loses its efficiency in low temperatures and dry atmosphere, the control of these two parameters is critical. In that way, the temperature and the relative humidity inside of the chamber should be kept between 24°- 38°C and 60-80%, respectively.
To complete the procedure, the time of exposure to the disinfecting agent should be of 20 minutes. Moreover, it is crucial to circulate the gas in order to make sure that surfaces of the eggs shell will have contact with the disinfecting gas. After this procedure, the gas should be expelled in a safe way. Finally, in order to ensure the efficacy of the whole process, the hatching eggs should be placed on wire racks, in wire baskets or on cup-type egg flats stacked in a manner that will permit air circulation and exposure to the formaldehyde gas.
Nevertheless, during the fumigation process, safety measures are critical and must be followed carefully. Some of these precautions include avoiding the use of plastic or polyethylene containers as the heat generated by the chemical reaction could set fire in those materials. Besides, to avoid possible fire hazards, the containers should slope outwards. It is also important to use containers large enough so that the two chemicals occupy no more than one quarter of its total capacity (volume). Preferably, the container should have a capacity of at least ten times the volume of the total ingredients. Furthermore, the operator is advised to use a mask in order to prevent irritation of the eyes and nose.
Even though formaldehyde has always been used as the product of choice for a long time, it remains the disinfectant of choice for hatching eggs (Scott and Swentnam, 1993). In the hatcheries, no embryonic mortality is observed under good practices of use (Scott et al., 1993). Moreover, used as fumigation, formol vapors show real efficacy to destroy microorganisms on the shell, the box, the hatching machines or every material used (only if, obviously, they are previously cleaned). Another explanation is nevertheless its relative efficacy in presence of residual organic matter. Besides, it seems that few cases of resistance of micro-organisms against formol have been found (Furuta, K., Maruyama, S., 1991).
However, research is no longer focusing on this molecule since, in some more regulated countries, it will belong to the past since the International Agency for Research on Cancer (IARC / WHO) pointed this molecule as carcinogenic for human beings. Several countries are thus looking for alternative solutions for egg disinfection, to replace the undesirable characteristic of this disinfectant, but keeping its efficacy.
Table 3: Fumigation Procedure at the Hatchery
^ Top page
.
2.2.2 Egg-Spraying
As for any disinfecting procedure, the spray method has to follow some requirements in order to proper sanitize the hatching eggs:
- Selection and dilution of the disinfectant: besides some other criteria related to the activity of the disinfectant (cf table 3), do not use any compound which can impair the exchange of gases through the eggshell.
- Correct application: to kill as many organisms as possible the disinfecting solution has to be applied in a manner which will thoroughly wet the shell surface. Undoubtedly, this is one of the limitations of this method of disinfection as it depends on the proper spraying and, at the farm level, it is not unusual to verify that the bottom of the eggs were not reached.
- In very cold whether, in order to avoid any thermal shock, it is advisable to heat up the water before preparing the disinfecting solution and spraying it on the eggs.
^ Top page
.
2.2.3 Egg-Dipping
Figure 2 & 3:Illustration of manual dipping with dirty eggs remaining
This method must be carried out very carefully. The temperature, for the reasons seen before, has to be monitored strictly to avoid any risk of temperature shock. Especially after dipping dirty eggs, the dirtiness remains in disinfectant solution and it can induce:
- lower activity of disinfectant
- contamination of other batches of eggs.
In order to avoid these problems, a pre-selection of the eggs before dipping is required (do not dip dirty eggs) and change the disinfecting solutions often (at least once a day).
^ Top page
.
2.3 Implications of Improper Sanitizing
Proper selection and use of a sanitizer is essential to good sanitation management and can prevent additional problems in the hatchery, especially when we the bacterial population on one eggshell can reach 300,000 CFU instead of about 300 on a sanitized shell (Wineland & Carmen, 2007). Since most incubators have greater than a 40,000 egg capacity, thousands of eggs and chicks could become contaminated if an infected egg explodes, breaks or becomes cracked inside the incubator. The proper practice of good management strategies will prevent microbial problems and aid in the production of quality chicks.
^ Top page
.
CONCLUSION
The disinfection of the eggs, even if compulsory in an economical view, must be reminded only as a way to secure this process, because it is unable to fix all the mistakes done before. In general, hatchery can indeed only maintain the quality of the eggs, not improve it.
But at the end of the day, the quality of the DOC will be assessed at the slaughter house, through the economical performance of the hatched flocks. And as a central link between multiplication level and production level, the hatchery has to ensure the best potency of production for the level below. Egg disinfection is only a non-sufficient but compulsory part of the DOC quality, and as such it has to follow accurate guidance. As a consequence, frequent check-up have to be carried out closely with the help of new laboratory tools. This is the evolution, especially with the progressive replacement of formalin by other products needing to be assessed for such a critical use.
^ Top page
.
ACKNOWLEDGEMENTS
Ceva Animal Health Asia would like to acknowledge Dr. Mahatma Arafat (DVM, CEAV Aviculture) for his kind technical assistance with this paper.
.
REFERENCES
_ Board R.G., Ayres J.C., Kraft A.A., Forsythe R.H., 1964: The microbiological contamination of egg shells and egg packing materials. Poult. Sci., 43, 584-595.
_ Bruce J., Drysdale E.M., 1994: Trans-shell transmission in Microbiology of the Avian Egg. Pages: 63-91.Eds. Chapman and Hall, London, England.
_ Code sanitaire pour les animaux terrestres, OIE, 2006.
_ Faruta K., Maruyama S., 1991: Bacterial contamination of eggs during incubation and hatching and of fluffs of newly hatched chicks. Br. Poultry Sci. 22:247-254.
_ Lock J.L., Dolman J., Board R.G., 1992: Observations on the mode of bacterial infection of hen’s eggs. FEMS Microbiol. Letters 100:71-74.
_ Marris. P, 1986: Efficacité de désinfectants sur la contamination des oeufs. Bull Rech vet, 17 (2) 123-128.
_ Sparks N.H.C and Burgess A.D., 1993: Effect of spray sanitising on hatching egg cuticle efficacy and hatchability. British Poultry Science 34: 655-662.
_ Scott T.A., C. Swetnam, 1993: Screening sanitizing agents and methods of application for hatching eggs. I. Environmental and User Friendliness. Journal of Applied Poultry Research. 2:1-6.
_ Scott T.A., C. Swetnam and R. Kinsman, 1993: Screening sanitizing agents and methods of application for hatching eggs. III. Effect of concentration and exposure time on embryo viability. Journal of Applied Poultry Research 2: 19-25.
_ Thermotes, Lies, 2003: effective hygiene within the hatchery. International hatchery practice, volume 20, N° 5.
_ Wineland M., Carmen C.: Spray sanitizing hatching eggs. North Carolina State University: http://www.ces.ncsu.edu/depts/poulsci/
^ Top page
.
...
.
ANNEXE I: Method Using Formalin As Fumigation (OIE – Code for Terrestrian Animals)
METHOD 1
a. Concentration A
53 ml formalin (37.5%) and 35 g potassium permanganate per m³ of space.
This can be expressed as:
5.25 oz by volume (148.5 ml) formalin (37.5%) and 3.5 oz by weight (98 g) potassium permanganate per 100 ft³ (2.8 m³) of space.
b. Concentration B
43 ml formalin (37.5%) and 21 g potassium permanganate per m³ of space.
This can be expressed as:
4 oz by volume (120 ml) formalin (37.5%) and 2 oz (60 g) potassium permanganate per 100 ft³ (2.8 m³) of space.
c. Concentration C
45 ml formalin (40%) and 30 g potassium permanganate per m³ of space.
This can be expressed as:
4.5 oz by volume formalin and 3 oz potassium permanganate per 100 ft³.
d. Procedure
Fumigation of hatching eggs and equipment should be carried out in a special chamber or in a room or building constructed of impermeable material which can be made as airtight as possible. A fan is necessary to circulate the gas during fumigation and to expel it after fumigation is completed.
The total volume of the room is determined accurately from the internal measurements. The space occupied by trays, or eggs, or articles to be fumigated, is to be disregarded. The quantities of materials required are based on the total volume.
Place in the centre of the floor, one or preferably several large metal basins, metal trays or containers of earthenware, enamelware, asbestos or other non-inflammable material.
An electric or hot water heater should be available in the chamber to maintain the temperature at 75°-100°F (24°-38°C). Water pans or other equipment should be available to provide a relative humidity of 60-80%.
Place required amount of potassium permanganate into the containers BEFORE adding the formalin.
Pour the required amount of formalin onto the potassium permanganate in the containers.
Leave the chamber as quickly as possible and close the door. Some operators may wish to use a gas mask when pouring the formalin into the containers.
The door of the chamber should be securely closed and permanently labelled to prevent accidental opening.
The fans should be operated to circulate the formaldehyde and the fumigation time should be 20 minutes.
After 20 minutes, the gas should be expelled through a controlled vent leading to the outside of the building.
The door may be opened to facilitate expelling the formaldehyde to the outside.
METHOD 2
An alternative method to the above is to use formaldehyde gas produced by the evaporation of paraformaldehyde. Proprietary preparations are available and the operation is carried out by placing the requisite amount of powder on a pre-heated hot plate.
In this method it is necessary to ensure that the relative humidity of the chamber is sufficiently high (60-80%).
Ten grams of paraformaldehyde powder or pellet is used per m³ of space.
^ Top page
.
ANNEXE II: Major Group of Disinfectants and Characteristics
GROUPS AND CHARACTERISTICS | ADVANTAGES | INCONVENIENCE | ||
|---|---|---|---|---|
HALOGENS | 1. Chlorinated products | - Sodium Hypochlorite - Chloramine - Sodium isocyanurate - Very common in agroalimentary industries | - Broad spectrum - Moderated cost - Low toxicity | - Bad stability (heat, light) - High sensitivity to organic materials - Activity strongly linked to pH - Irritant for eyes |
2. Iodinated products | - Very good activity - Cheap - Action at cool temperature - Low toxicity | - Coloration of materials - Corrosives - Bad conservation | ||
ALDEHYDES | Formol | The health concern with formol enhances the actual use of glutaraldehyde. | - Broad activity spectrum - Cheap - Not really affected by pH | - Not quick action - Low penetration |
Glutaraldehyde | - Slow action - Is toxic and dangerous - Iritant smell | |||
QUATERNARY AMMONIUMS | Widely active on Gram (+) bacteria and moulds. Often associated with aldehydes to broaden their action on Gram- bacterias and improve virucid strength. | - Moisturizing power (a bit detergent), - Non-corrosives - Good degradability - Very good activity | - Incompatibility with anionic products - Susceptibility to organic materials… often balanced by associationwith aldehyde.
| |
PHÉNOLS AND DERIVED | If phenol use in itself is very limited due to its strong toxicity, derived phenols are often used as disinfectants. Mainly : - Chloro- 4 -Méthyl 3 phénol - Benzyl- 4 -chlorphénol | -Good bactericidy -Low susceptibility to organic materials | Many inconvenience: - Can induce skin lesions (percutaneous) - Low virucid activity - Susceptibility to water - Non-compatible with cationic products - Ecologic concerns (biodegradability) - Banned in many agroalimentary Industries - Irritant smell | |
PEROXYDES | Two very common ones: - Hydrogen peroxyde - Peracetic acid | - Good efficacy | - Very unstable (temperature, organic material, …) - Dangerous to manipulate | |
^ Top page
If you need to download this article, please do not hesitate to contact us!

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam