...
-->Introduction
-->Some Advantages Expected From In-Ovo Vaccination
-->Conclusion
...
BASIC ASPECTS OF IN-OVO INJECTION IN COMMERCIAL HATCHERIES
By Fabio Moreira de Souza– Poultry Equipment International Manager
Ceva Santé Animale – Libourne, France
.
Introduction
Although the In-ovo vaccination has been widely used in many countries all over the world for over 20 years, many questions still surround this technology.
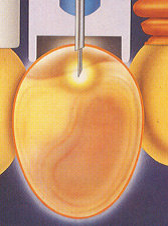
Image 1In-ovo vaccination technology
To achieve the best of In-ovo vaccination not only a good machine is essential. To understand the relation between chick quality and In-ovo injection, it is important to realize the factors directly influencing In-ovo application. Some of those factors have expressive influence in the final expected result: a good and effective vaccination.
Chick Quality and In-ovo vaccination are intrinsically related when we discuss many factors such as: Embryonic age and hatchability, site of injection, vaccine preparation and immune response, hatchery hygiene and sanitation.
In this article some of the factors and basic aspects related to In-ovo injection will be discussed.
.
In-Ovo Injection History
The In-ovo vaccination was first proved to be efficient for Marek’s vaccine by experiments performed by Sharma and Burmester in 1982. These authors demonstrated that chicks vaccinated In-ovo at 18 days of embryo development against Marek’s Disease had better protection against virulent MD challenge carried out at 3 days of age as compared to those chicks vaccinated at hatch. At seven days of age, the both groups (vaccinated at hatch or In-ovo) induced equivalent level of protection.
At that time In-ovo vaccination was only a new idea, but today it is being used in commercial applications worldwide in the poultry industry. The basic laboratory concept of In-ovo vaccination, initially used for vaccination against Marek’s disease, has expanded and today we can find machines capable of inject up to 70,000 eggs per hour.
.
Embryonic Development
To know the stage of development of the embryos is essential to determine the correct time to achieve the best hatchability and to perform In-ovo injection. Moreover, embryonic development is directly associated to many aspects of the incubation process.
Before In-ovo injection is taken into consideration, it is important to remember that many studies have shown that eggs transferred too early (around day 17 of incubation) from Incubators to Hatchers tend to have their hatchability reduced when compared to eggs transferred later (day 18 or 19), whether they are injected or not. The differences (water loss, humidity, heat and gas exchange) in the environment of these two types of equipment are the main responsible for differences in hatchability.
Normally, a positive effect on hatchability is observed if the embryo remains in the incubator for longer time, and is transferred to the hatchers later. Also, difference in embryonic development may be observed in different types of incubators due to their different water loss and temperature standards, and to determine the correct time for In-ovo injection - to achieve maximum hatchability and good immune response against diseases - the physiological characteristics of the embryo are much more important to be considered than the number of hours of incubation.
The optimal timing forIn-ovoInjection is normally recommended to be between 17.5 and 19.2 days of Incubation (count starts from Egg Set Time). The lower limit – 17.5 days – is normally related to the period the yolk sac is starting to enter the abdomen and the head of the bird is positioned under the right wing. The upper limit – 19.2 days – is limited by the percentage of eggs presenting external pipping, since the presence of multiple holes in the egg can contribute to egg cracking during transfer process. Eggs cracked on the side or the small end generally result in embryos stuck to the exposed (and dried) membrane or the egg shell, therefore reducing hatchability. It is not recommended to perform In-ovo injection in flocks with more than 1% of pipped eggs.
.
Sites of Injection
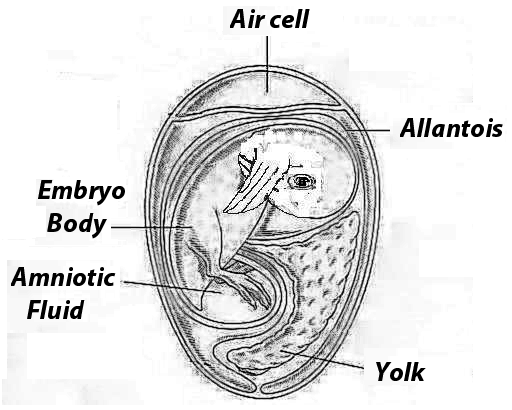
We can consider that there are 5 basic compartments on an egg during the final stage of incubation: Air Cell, Allantoic sac (waste), Amniotic sac, the Embryo itself and the Yolk sac.
Image 2 Five sites of injection on an egg
- The air cell which is basically filled with gas.
- The allantoic sac which is filled with fluid and it stores what it is usually referred as the waste inside the egg.
- Amniotic sac which is composed by amniotic fluid and the embryo body.
- The embryo itself which is located inside the amniotic sac.
- The yolk sac which is also inside the amniotic sac.
Any one of those compartments can be accessed by the needle of the In-ovo injection machine. However, in order to achieve and maximize immune response by In-ovo vaccination, it is essential to assure that the correct compartment inside the egg is accessed.
Due to its fast development in this last stage, specially when we consider the In-ovo injection window (as mentioned before, between 17.5 and 19.2 days of incubation), it is important to realize that these compartments can change fast as they are utilized by the embryo. It is also essential to recognize that the specific compartments are responsible for different support during the development of the embryos, and the placement of vaccines and/or other compounds into those compartments may allow or limit their absorption by the embryo.
The position of these compartments inside of the egg, and more specifically, the position of the embryo depend on its stage of development.
.
Immune Response
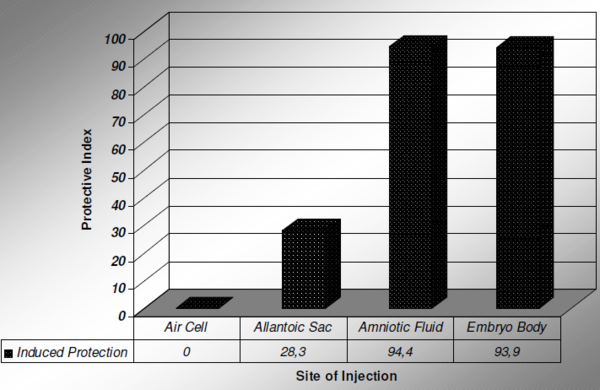
Scientists of the University of California at Davis (Wakenell et al., 2002) conducted some trials in order to evaluate the ability to induce immune response when a MD vaccine is delivered in different compartments of the egg (Figure 1).
Figure 1 Immune Response (MD Vaccine) related to site of injection
The results have shown that the deposition of MD vaccine in the air cell or the allantoic sac does not induce effective immune response. Those studies reported that MD vaccine injected into the air cell resulted in no protection of the bird while injection in the allantoic sac induced only 28.3% of protection. Injection in the amniotic fluid or in the embryo body resulted in about 94% protection of the vaccinated birds.
In conclusion, in order to be able to induce effective immunity against the Marek’s Disease, from those 5 basic compartments described above, only 2 of them have the ability to promote positive immune response: the amniotic fluid and/or the embryo itself. The vaccine must be delivered deep enough inside the egg to reach the amniotic sac (amniotic fluid and embryo).
It is expected that injections in the initial stage of the In-ovo injection window (closer to 18 days of incubation), most of the eggs would be normally injected in the amniotic fluid. When the vaccine is delivered in the amniotic fluid, the substance will be swallowed by the embryo prior to hatch. On later injections (around 19 days of incubation), as the embryo is more mature, a bigger percentage of embryos would receive the injection in their body (expected to happen in the right breast). This injection in the embryo body is normal and acceptable, however excessive depth penetration can result in embryo damage.
As noted, the site of injection is a critical point to induce effective immune response and precision is needed to allow the vaccine to be delivered in the correct site inside the egg, therefore resulting in effective vaccination.
.
Some Advantages Expected From In-Ovo Vaccination
Healthier birds: earlier exposure to vaccines allow the birds to develop earlier immunity.
Reduced chick stress:In-ovo injection reduces chick handling and stress from manual injection at hatcheries after they hatch.
Precise, uniform injection:In-ovo injection ensures uniform process, delivery of consistent volumes and accurate dosage to nearly 100% of eggs.
Reduced labor costs:In-ovo injection systems result in a reduction in the need for labor when compared to the vaccination of birds on day of hatch.
Needle Sanitation: with the individual sanitation of each needle after each injection the risks of contamination and disease spread are minimized when compared to manual post-hatch injection.
.
Conclusion
In-ovo injection has become an important tool to administer vaccines in the hatcheries. As it was aforementioned, everything started around 25 years ago with Marek’s Disease vaccine and today there are several other products to be injected through this equipment. Products like Cevac® Transmune IBD, Vectormune® HV-IBD or Vectormune® HVT-ND are already commercially available and many others are being developed, clearly demonstrating an even brighter future for this technology.
Nevertheless, some basic pre-cautions must be taken into account in order to achieve the best results with this tool. Good sanitation of the hatchery, proper disinfection of the hatching eggs are among these special cares. Furthermore, precise maintenance of the machine is compulsory. By ensuring that these procedures are properly followed, companies can sure benefit from this interesting technology.
.
REFERENCES
Avakian, A., P.S.Wakenell, T.Bryan, J.L.Schaeffer, C.J.Williams, and C.E.Whitfill, 2002. In-ovo Administration of Marek’s Disease Vaccine: Importance of Vaccine Deposition Site in the Fertile Egg. Proceeding of the 51st Western Poultry Disease Conference, May 1- 4, 2002, pp.119-121, Puerto Vallarta, Mexico.
Meijerhof, R., “Maximising in-ovo vaccination success”
Phelps, P., “The In-ovo Administration of Antibiotics into Broiler Eggs at Transfer”, Ph.D Dissertation, North Carolina State University, 1995.
Sharma, J.M and Burmester, B.R., Resistance to Marek’s Disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus, Avian Diseases, v. 26, p. 134-149, 1982
Wakenell, P.S., T.Brian, J.Schaeffer, A.Avakian, C.Williams, and C.Whitfill, 2002. Effect of In-ovo Vaccine Delivery Route on Herpes Virus of Turkey/SB-1 Efficacy and Viraemia. Avian Disease 46(2); 274-280.
Williams, C.J., “In-ovo Vaccination and Chick Quality”, International Hatchery Practice – Volume 19 Number 2.
Williams, C.J., “In-ovo vaccination for disease prevention”, Pfizer/Embrex Web Home Page
If you need to download this article, please do not hesitate to contact us!

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam